









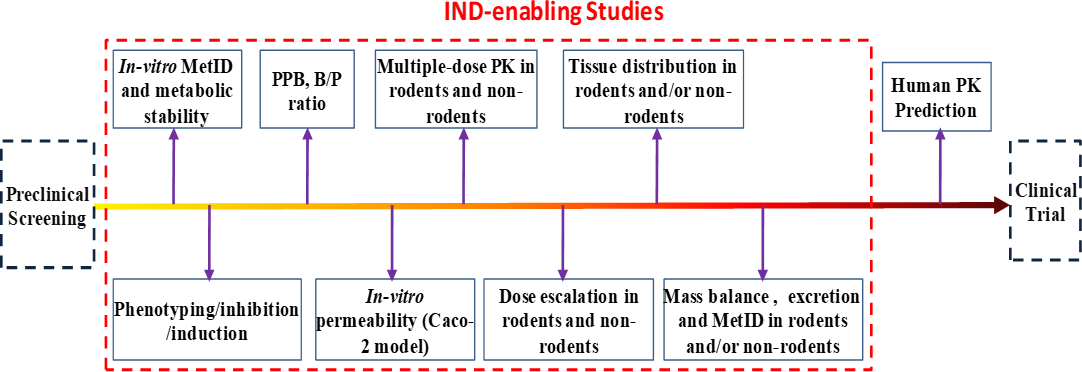
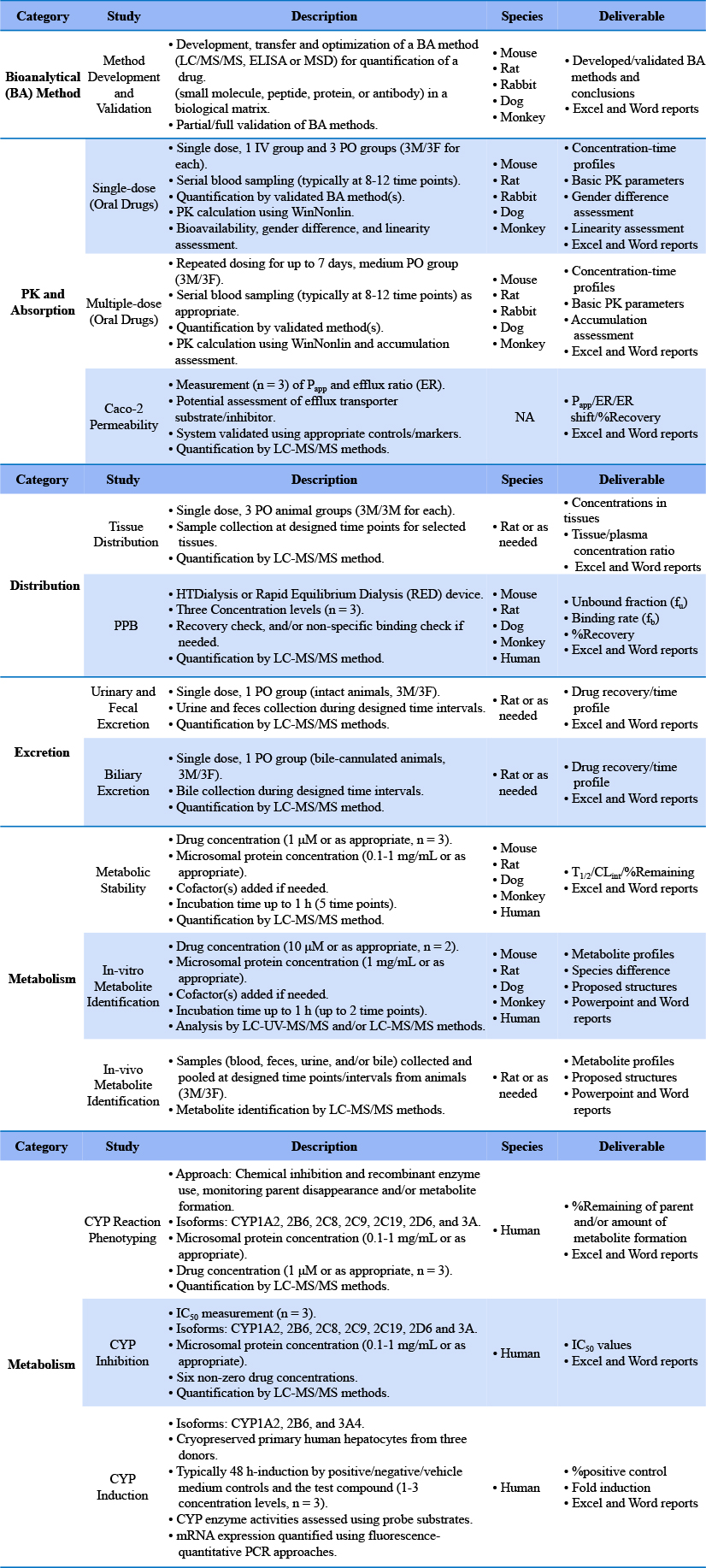
The term IND (investigational new drug) refers to a new drug to be evaluated in clinical trials. An IND application is a request for authorization by the FDA/CFDA to administer an investigational drug to humans. Such an application is composed of sets of IND-enabling studies, among which DMPK studies represent an essential part. The DMPK study contents required by the FDA/CFDA in their IND approval processes are quite similar though there are minor differences. Over the past six years, 3D BioOptima has completed over 400 IND-enabling DMPK package studies for our clients (6 for BeiGene alone), with all our work passed regulatory inspections. Over 300 of them have been approved to enter into clinical trials by CFDA and over 200 of them by US FDA and Australian TGA.








You can contact us through the right side, we will assign a professional consultant to answer your questions.


The SPF and general animal rooms, with more than 600 square meters, can perform experiments on mice, rats, dogs, monkeys, rabbits, small pigs, and many other genera.
Oral gavage, sublingual administration, injection, topical application, whole blood collection, excreta collection, tissue collection, eye tissue collection, cerebrospinal fluid collection, bile duct intubation, etc.
A strict quality management system is in place to ensure the reliability of data and validity of research.

There are more than 400 sets of various instruments such as 16 sets of LC-MS/MS systems, 2 sets of MSD, analytical balances of 1 part in a million and 1 part in 100,000, respiratory anesthesia machines, IVC systems, various incubators, tissue homogenizers, centrifuges and so on.
In vitro metabolic studies, including metabolic studies based on liver microsomes, hepatocytes, liver S9, CYP enzymes, etc;
Analysis of biological samples for PK, tissue distribution and excretion experiments, development and validation of analytical methods, parameter calculations, etc.
The laboratory follows GLP (Good Laboratory Practice) and ISO standards to ensure data reliability and accuracy.

More than 100 bead cell banks have been established.
In vitro cell proliferation inhibition, Caco-2 permeability, and hepatocyte-related experimental studies can be performed.
The laboratory has a cell bank of more than 100 beads. The laboratory strictly follows the experimental practice to ensure the accuracy and reproducibility of the data.

Customer first, customer satisfaction!

Customer First, Customer Satisfaction!