









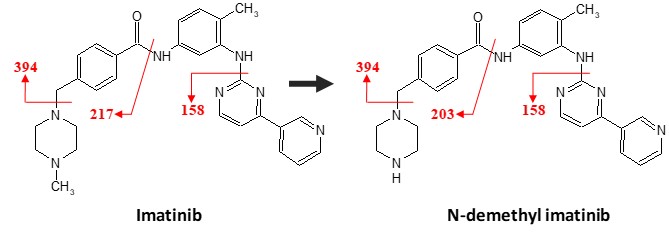
美国FDA于2008年发行的关于MIST的工业指南强调了应该在新药研发的过程中尽早考虑代谢物在药物整体毒性中的作用。而利用体外代谢模型进行的代谢物鉴定工作则是药物研发工作者们出于该目的而在早期开展的必要研究步骤。借助最先进的仪器和软件(如AB SCIEX QTRAP 5500和LightSight代谢物鉴定软件),圣苏新药公司经验丰富的科学家们能够在各种属中进行全面的代谢物谱分析和结构解析。







You can contact us through the right side, we will assign a professional consultant to answer your questions.


The SPF and general animal rooms, with more than 600 square meters, can perform experiments on mice, rats, dogs, monkeys, rabbits, small pigs, and many other genera.
Oral gavage, sublingual administration, injection, topical application, whole blood collection, excreta collection, tissue collection, eye tissue collection, cerebrospinal fluid collection, bile duct intubation, etc.
A strict quality management system is in place to ensure the reliability of data and validity of research.

There are more than 400 sets of various instruments such as 16 sets of LC-MS/MS systems, 2 sets of MSD, analytical balances of 1 part in a million and 1 part in 100,000, respiratory anesthesia machines, IVC systems, various incubators, tissue homogenizers, centrifuges and so on.
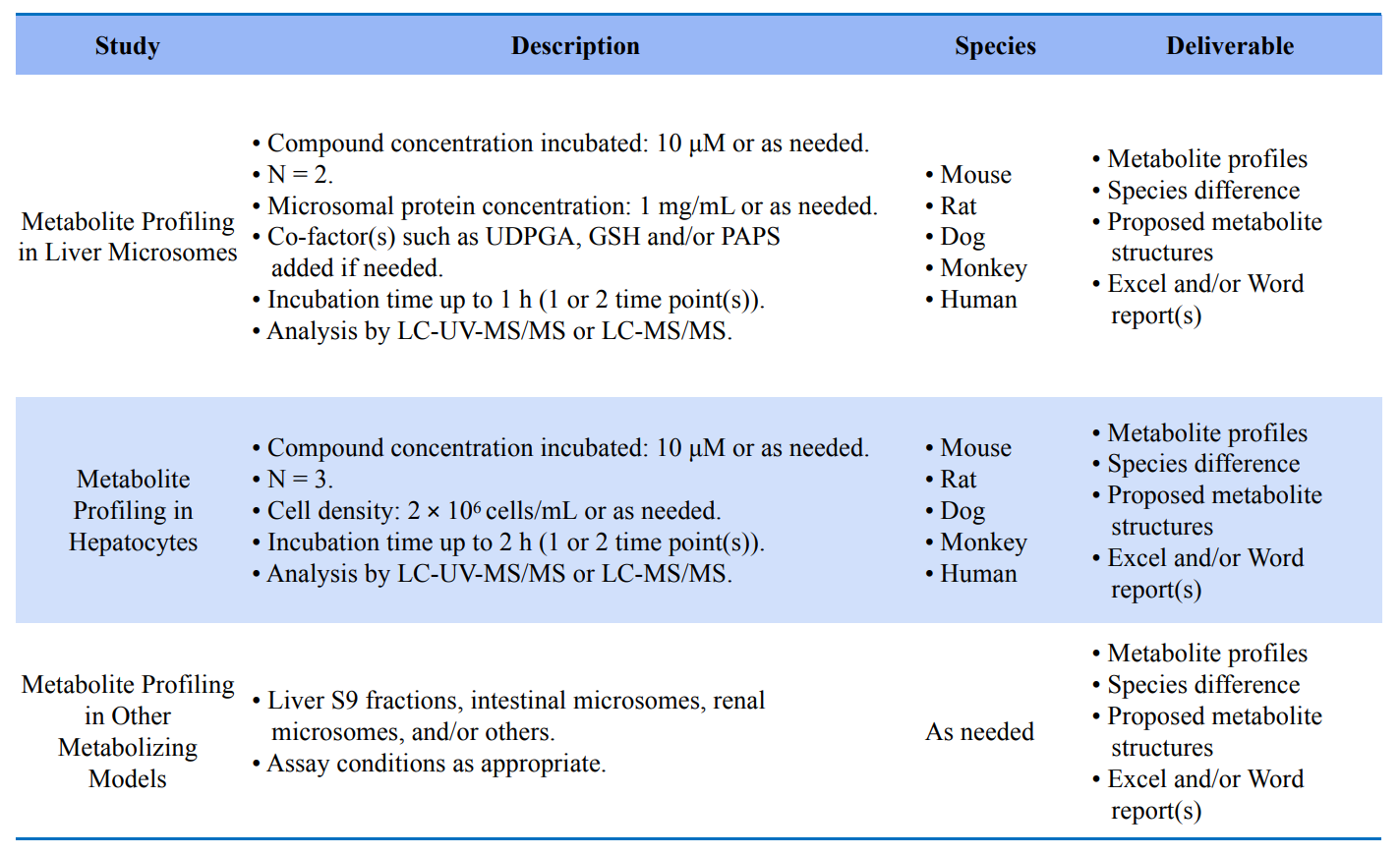
In vitro metabolic studies, including metabolic studies based on liver microsomes, hepatocytes, liver S9, CYP enzymes, etc;
Analysis of biological samples for PK, tissue distribution and excretion experiments, development and validation of analytical methods, parameter calculations, etc.
The laboratory follows GLP (Good Laboratory Practice) and ISO standards to ensure data reliability and accuracy.

More than 100 bead cell banks have been established.
In vitro cell proliferation inhibition, Caco-2 permeability, and hepatocyte-related experimental studies can be performed.
The laboratory has a cell bank of more than 100 beads. The laboratory strictly follows the experimental practice to ensure the accuracy and reproducibility of the data.

Customer first, customer satisfaction!

Customer First, Customer Satisfaction!